Patients with a MammaPrint Ultra-Low Risk signature have excellent outcomes regardless of age, clinical risk or treatment received
Prospective Validation of MammaPrint Ultra-Low Risk from the MINDACT trial1
MammaPrint® delivers significant guidance for endocrine management decisions for women with ER+, HER2-, lymph node negative breast cancer.
Published in the Journal of Clinical Oncology in January 2022, this study from MINDACT evaluated the long-term outcomes for patients classified as MammaPrint Ultra-Low Risk.
- With 1000 patients and a median follow-up of 8.7 years, the outcomes reported are from the largest prospective cohort to date.
- These patients have the best prognosis, as compared to patients with other classifications, regardless of clinical risk factors, treatment received or age.
- Identifying these patients may help further the de-escalation of treatment, avoiding unnecessary costs, toxicities and the risk of side effects.
Ultra-Low Risk outcome by Treatment
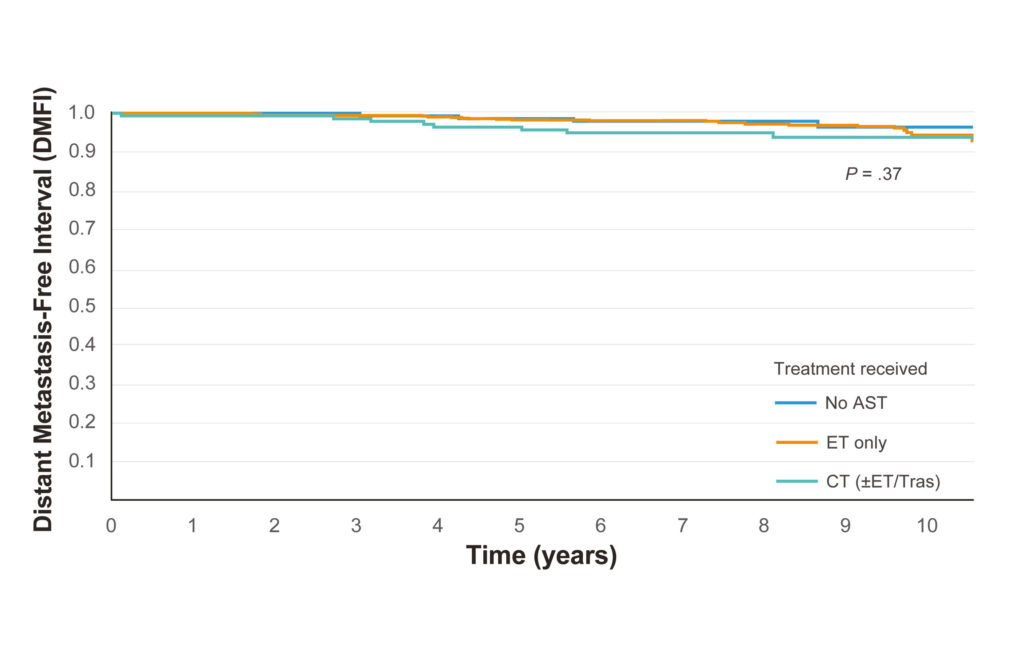
AST = Adjuvant systemic treatment; ET = Endocrine therapy; CT = Chemotherapy
8-yr DMFI outcomes of Ultra-Low Risk patients by treatment received
• No AST: 97.8%
• ET Alone: 97.4%
• CT+ET: 94.9%
• No significant difference (p=0.37)
Ultra-Low Risk outcome by Age
8-yr DMFI outcomes for Ultra-Low Risk patients by age
• <50: 95.6%
• >50: 97.6%
• No significant difference (p=0.44)
Ultra-Low Risk outcome by Clinical Risk
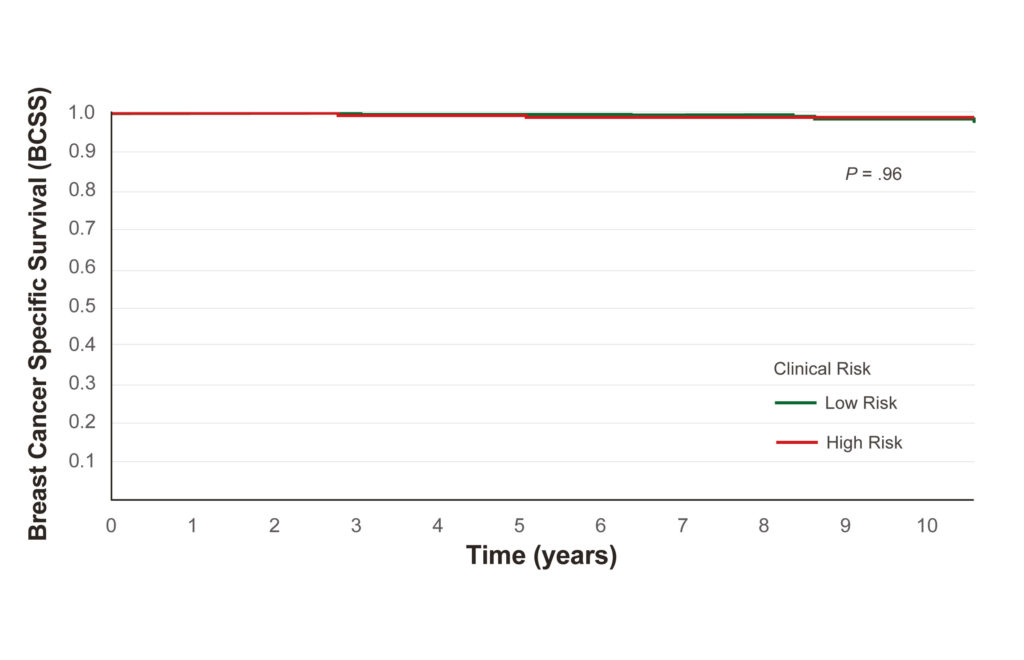
8-yr BCSS outcomes for Ultra-Low Risk patients by clinical risk
• Clin-Low: 99.7%
• Clin-High: 99.2%
• No significant difference (p=0.96)
(*MammaPrint Ultra-Low Risk is a subset within the Low Risk category.)
What is MammaPrint Ultra-Low Risk?
A threshold was established within the MammaPrint Low-Risk category to identify patients with an Ultra-Low Risk of distant recurrence.2
• Patients with a MammaPrint Index of >+0.355 are classified as Ultra-Low Risk.1
• Approximately 15% of all MammaPrint results are Ultra-Low Risk.1
Endocrine Therapy Management Implications
Extended adjuvant endocrine therapy treatment decisions:
• 25% of MammaPrint Low Risk patients are also Ultra-Low Risk, and can be candidates for endocrine therapy de-escalation.3
• The remaining 75% of patients are Low-Risk (Not Ultra-Low) and may significantly benefit from both standard and extended endocrine therapy.4
• In the NSABP B-42 analysis, Ultra-Low Risk patients had no significant benefit with extended letrozole therapy.4
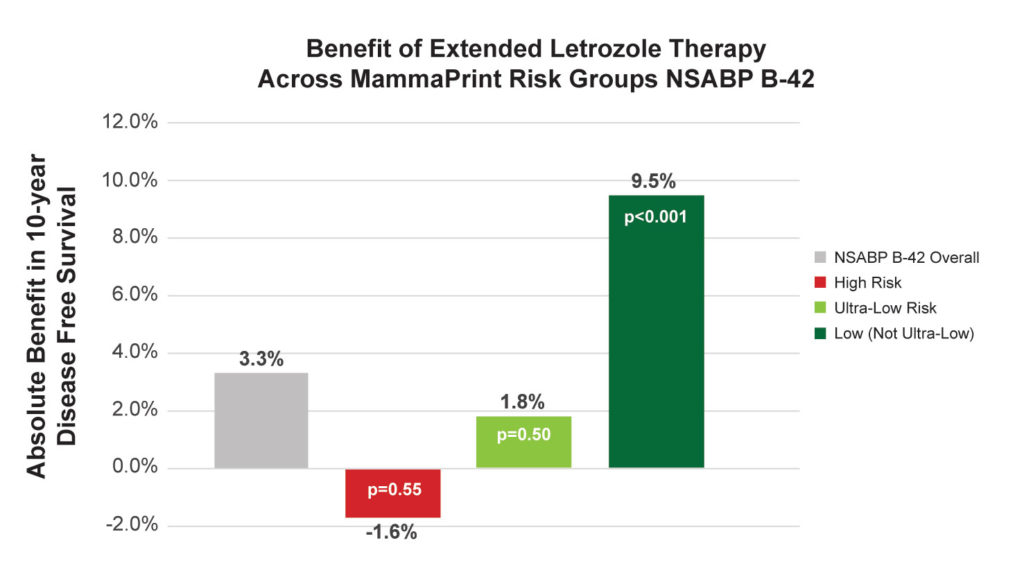
10-yr DFS absolute benefits of extended letrozole therapy
• NSABP B-42: 3.3%
• High Risk: -1.6%
• Ultra-Low Risk: 1.8%
• Low Risk: 9.5%
References
1. Lopes Cardozo JMN, et al; Outcome of Patients With an Ultralow-Risk 70-Gene Signature in the MINDACT Trial; J Clin Oncol. 2022 Jan 21
2. Delahaye LJMJ, et al: A breast cancer gene signature for indolent disease. Breast Cancer Res Treat 164:461-466, 2017
3. Esserman LJ, et al: Use of molecular tools to identify patients with indolent breast cancers with ultralow risk over 2 decades. JAMA Oncol 3:1503-1510, 2017
4. Rastogi P, et al: Utility of the 70-gene MammaPrint assay for prediction of benefit from extended letrozole therapy (ELT) in the NRG. Oncology/NSABP B-42 trial. J Clin Oncol 39, 2021 (suppl 15; abstr 502)

